SOLVED: The density of air can be calculated using the ideal gas law. If the molecular weight of air is taken to be 28.97 kg/kmol, estimate the air density at 300 K
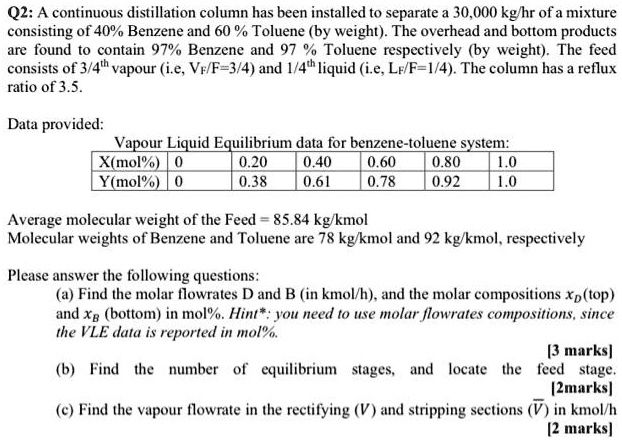
SOLVED: A continuous distillation column has been installed to separate a 30,000 kg/hr of a mixture consisting of 40% Benzene and 60% Toluene (by weight). The overhead and bottom products are found
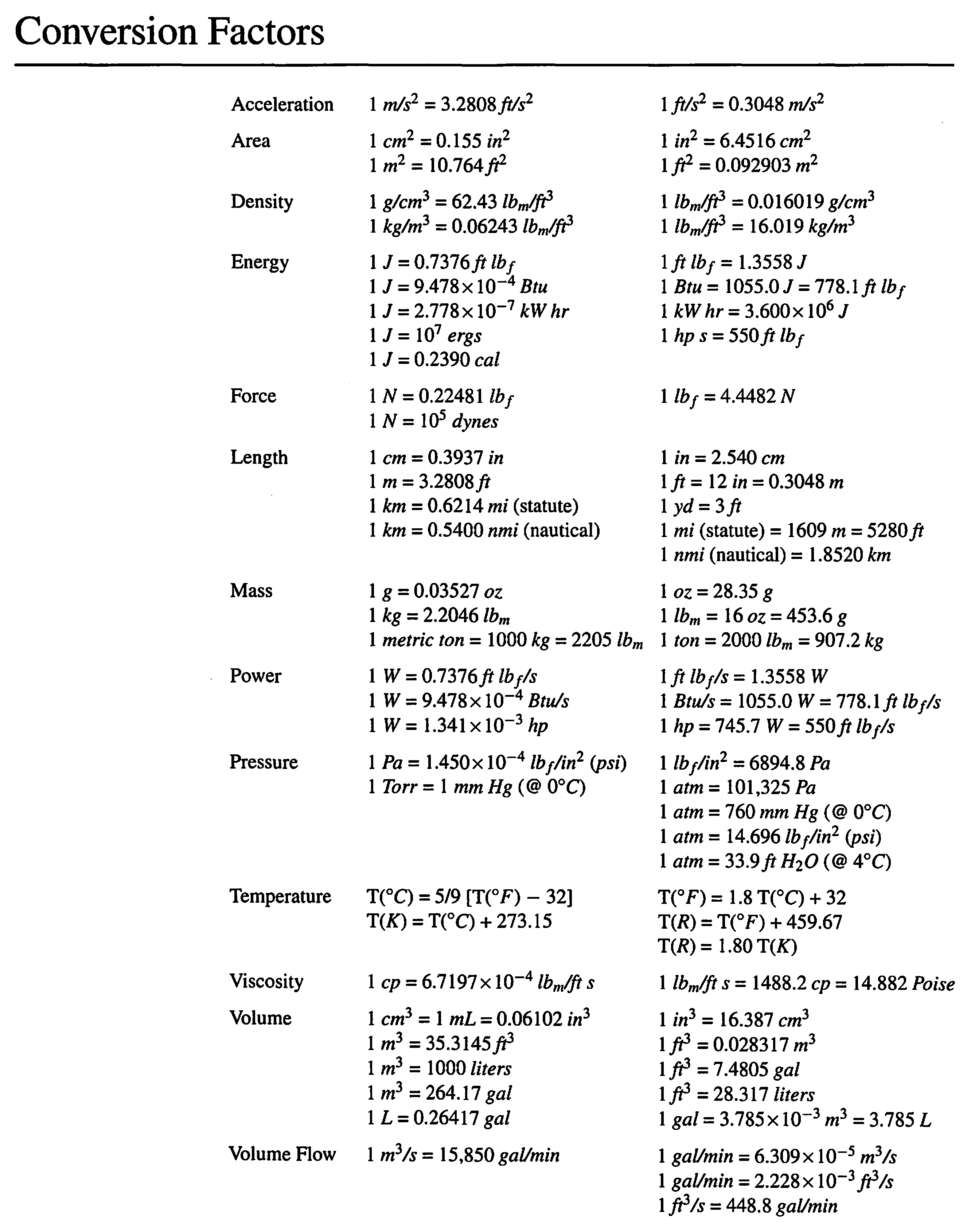
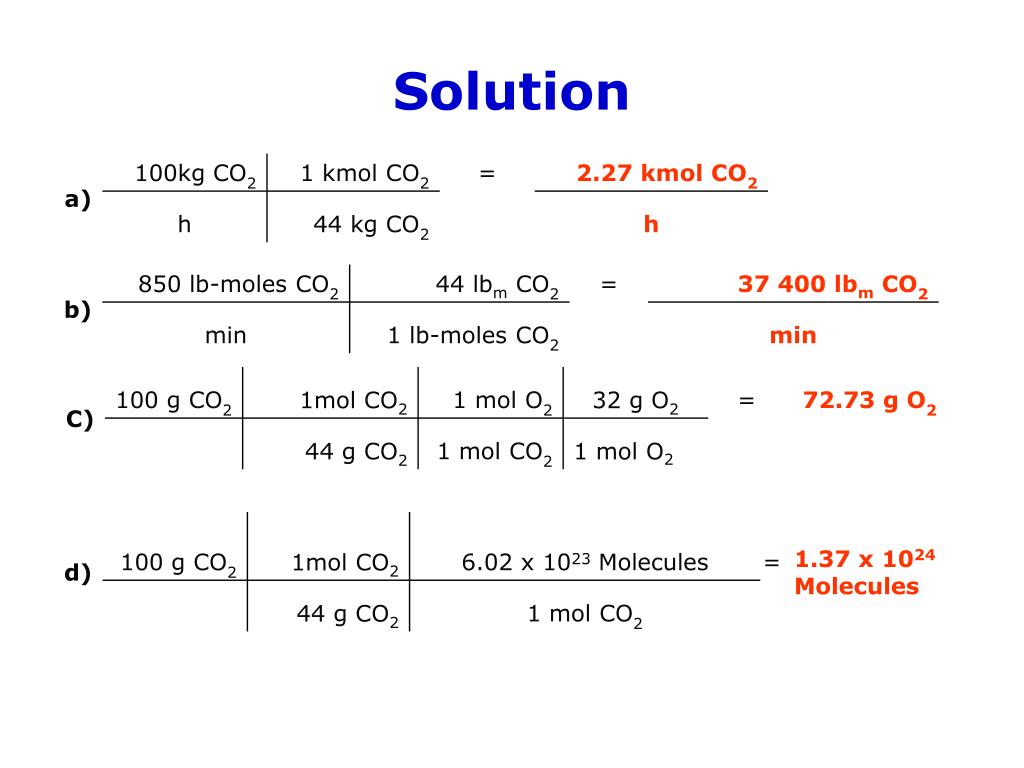
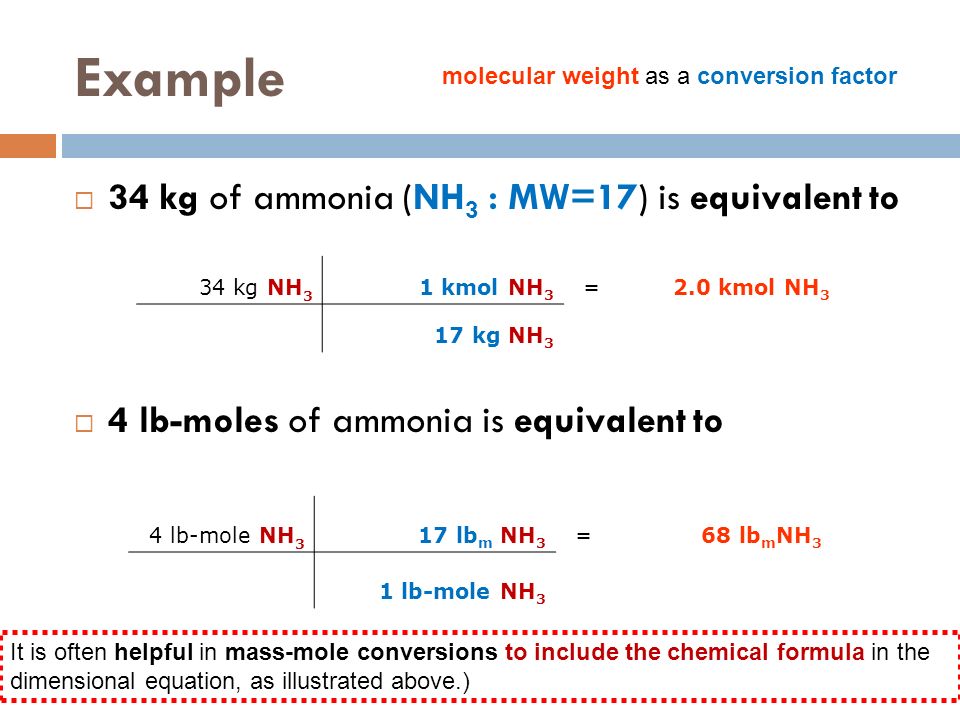
A gas contains 75.0 wt% methane, 10.0% ethane, 5.0% ethylene, and the balance water. (a) | StudySoup
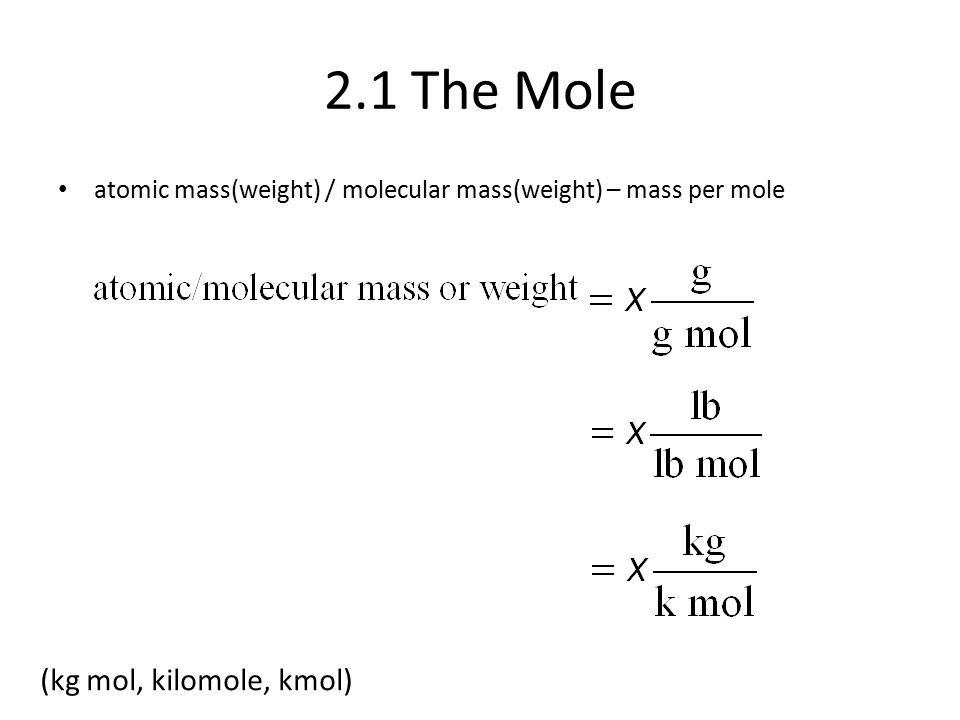
Lecture 2. Moles, Density, Specific Gravity, Fraction, Pseudo-Molecular Weight of Air, Concentration and Flow Rate. - ppt video online download

SOLVED: 7.Molality is a measure of the number of moles of solute in a solution corresponding to 1 kg or 1000 g of solvent. This contrasts with the definition of molarity which

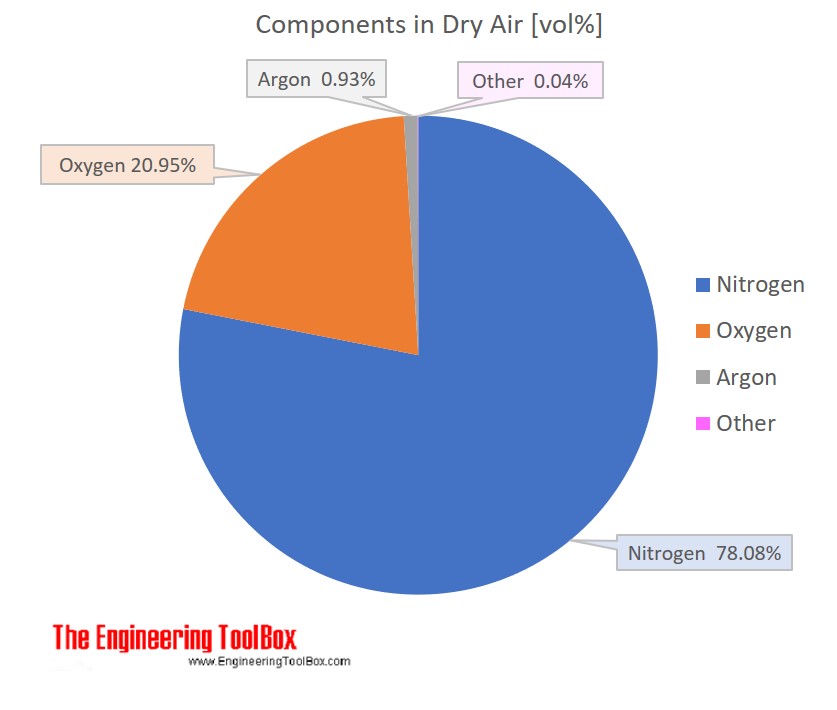
SOLVED: Molecular mass of air is 28.97 kg/kmol (Do NOT interpolate, use the closest property values in the ideal gas table). Show detailed work to receive credits. Your score is: For Instructor