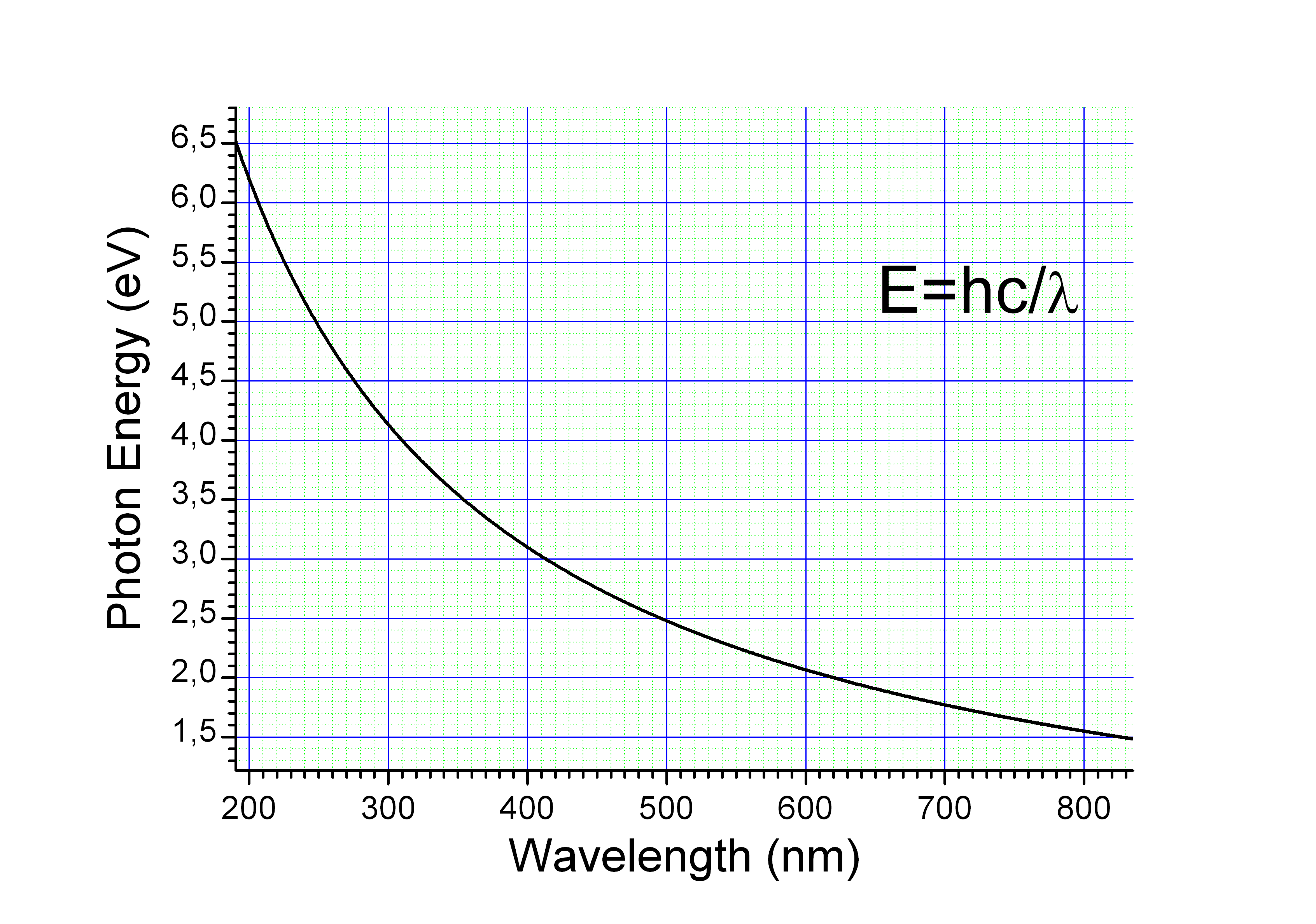
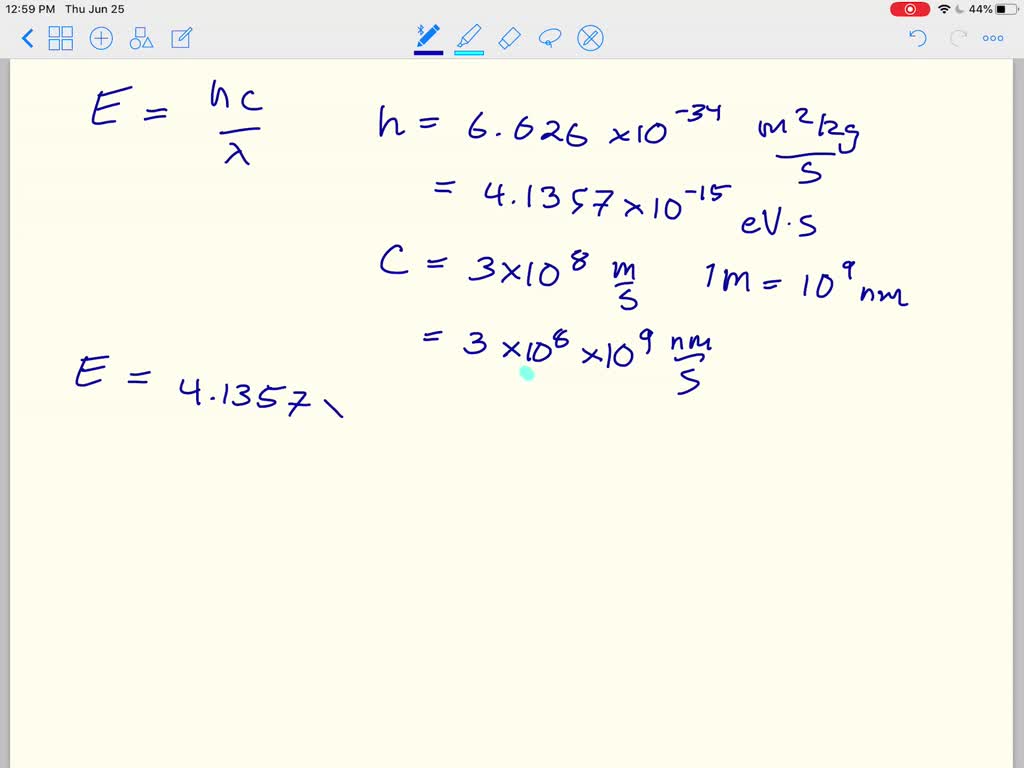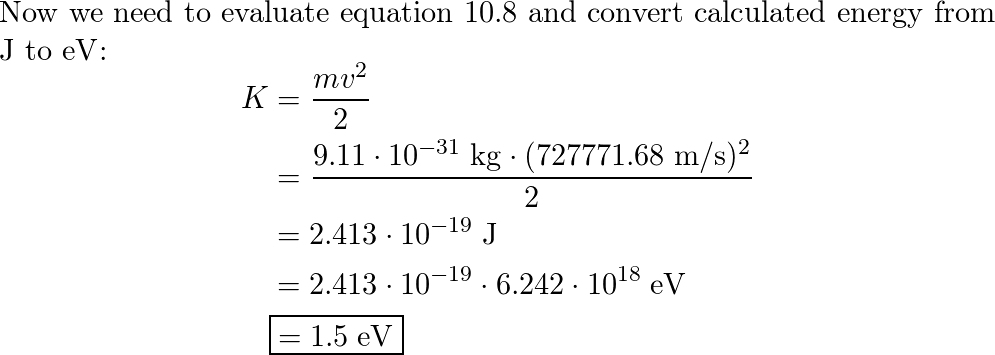Calculating particle properties of a wave Ch. 12 A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency. - ppt download

Get the Basics Right: Jacobian Conversion of Wavelength and Energy Scales for Quantitative Analysis of Emission Spectra | The Journal of Physical Chemistry Letters

Calculating particle properties of a wave Ch. 12 A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency. - ppt download